Homepage // Treatments / Laser Treatments / PicoSure Laser
PicoSure Laser Tatto Removal New York, NY
The PicoSure laser is a top choice in treating unwanted tattoos and pigmented lesions in New York.
- Downtime: Minimal
- Pain Level: Mild
- Results Duration: Long Lasting
Treats:
Unwanted tattoos, pigmented lesions, birthmarks, stretch marks, melasma
Side Effects:
Mild redness and swelling 1-2 days after treatment
Location:
Can be used on nearly any area of the face and body
Follow Up:
Some treatments require a series of sessions to achieve an optimal outcome
How Performed:
Handpiece delivers laser energy into the dermal tissue
Preparation:
Topical or local anesthesia administered prior to treatment
Procedure Type:
Laser
Expected Outcome:
Reduction or elimination of tattoo ink or pigmented lesion
Onset:
Results visible once skin heals after final treatment
Picosure laser

The Laser & Skin Surgery Center of New York® offers New York’s first Picosecond laser for tattoo removal, pigmented lesion removal, and acne scarring.
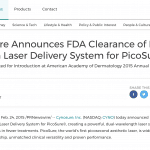
The FDA has cleared the PicoSure™ laser for tattoo and pigmented lesion removal. The approval is based on the clinical work performed at the Laser & Skin Surgery Center of New York® under the direction of Dr. Roy G. Geronemus, which has been published in the Archives of Dermatology.
The board-certified physicians at the Laser & Skin Surgery Center of New York have an extensive range of laser devices that allow us to customize all our patient’s treatments to ensure an optimal outcome. We have the experience and expertise to help you meet all your aesthetic goals with comfortable treatments and minimal downtime in most cases. Our team would be happy to meet with you to discuss your concerns and create a personalized treatment plan to keep you looking and feeling your absolute best.
How does the Picosure Laser Work?
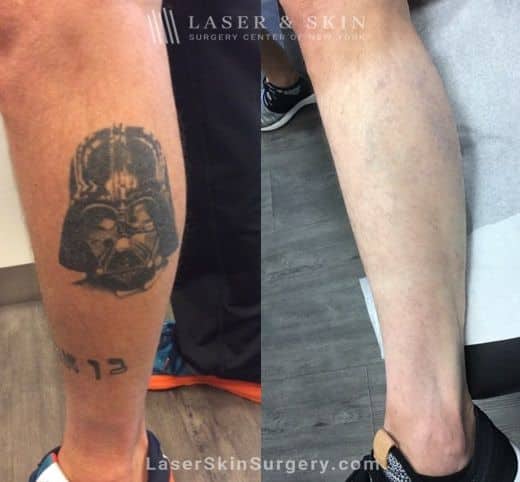
We harness our leading knowledge of the PicoSure™ laser, having published the first article on using this device on patients with tattoos. This laser uses short bursts of energy to rapidly clear almost all tattoos, especially the blue and green colors that have been difficult to remove by other lasers.
The clinical work performed at our center led to the PicoSure™ being approved by the Food and Drug Administration (FDA). In our clinical study of 22 subjects with multi-colored and recalcitrant tattoos, the PicoSure™ laser achieved more than 80% overall tattoo clearance over a two-week period. On average, 94% clearance was achieved for blue and green ink.
We continue to develop advances with picosecond laser technology as we are presently investigating two additional wavelengths for the device which we
anticipate will provide significant benefit to patients seeking tattoo removal.
What does the PicoSure Laser Treat?
Although the PicoSure laser is primarily used for tattoo removal, it can also be effective in treating the following:
- Acne scarring
- Age and brown spots
- Birthmarks
- Café-au-lait macules
- Melasma
- Nevus of Ota
- Stretch marks
To learn more about how PicoSure can treat your skin, checkout our latest webinar video.
When Will I See Results?
Results of a PicoSure treatment become evident as soon as the skin heals. If you require a series of treatment sessions, you will see full results after you recover from your final appointment.
How Long is the Recovery Time After Treatment?
There is no downtime after a PicoSure laser treatment. Side effects like swelling and skin redness should not be severe enough to keep you from your regular activities.
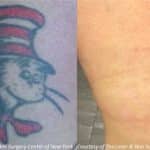
Real Patients Before and After photos
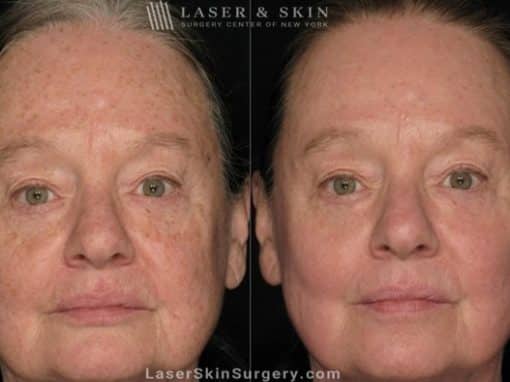
Laser Treatment for Actinic Keratosis on Female Patient
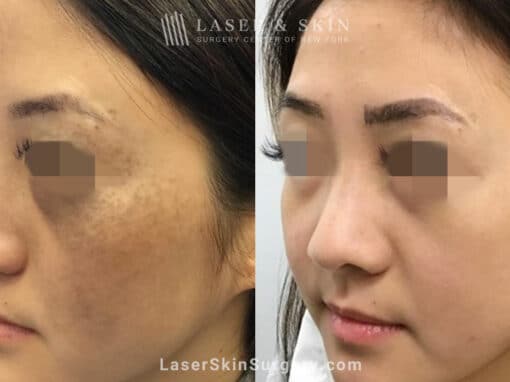
Picosecond laser to treat nevus of ota birthmark
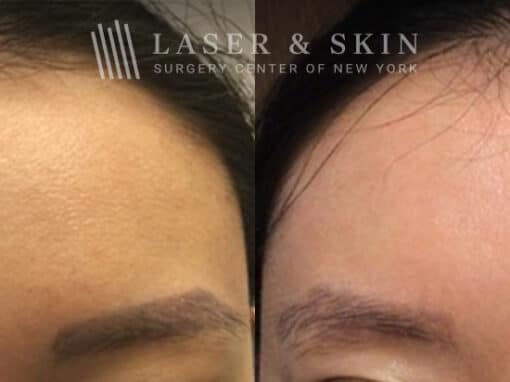
PicoSure laser to remove eyebrow tattoos
PicoSure Laser In The News
PicoSure Laser
Questions and Answers
Q: Is The PicoSure Laser Safe?
A: Yes, this device was FDA-approved in 2012 as the first of other picosecond lasers that have shown a strong record of safety and success. Our board-certified physicians have extensive experience using this device and will use their expertise to customize your treatment so it is as safe and effective as possible.
Q: How Many Treatments Will I Need?
A: That depends on what we are treating. Tattoos usually take a few treatment sessions to achieve full results, while other concerns may be successfully addressed in a single treatment session. When you meet with your doctor at your initial consultation, you will receive a treatment plan so you know what to expect moving forward.
Q: How Long Do Results Last?
A: In many cases, the results are very long-lasting, since the tattoo or mark is removed from the skin. However, results vary from patient to patient.
Q: Are There Any Side Effects?
A: The rapid pulse technology and the precision of the device mean fewer side effects than other laser treatments. Patients may experience mild swelling and skin redness for a few days after treatment. Bruising can also occur. None of these side effects should be severe enough to prohibit you from your daily activities.
Q: Am I A Good Candidate For PicoSure Laser Treatments?
A: This treatment is a good choice for patients that have tattoos or pigmentation concerns they would like to eliminate. The treatment works equally well for men and women and is appropriate for most skin tones.
Next, read about…
Scar Treatments
Scars are often unwanted reminders of medical conditions, injuries, trauma, or surgery. When they are noticeable, they can also lead to significant embarrassment and self-esteem. Fortunately, there are ways to treat scars today that do not involve incisions, anesthesia, and downtime.
Body Contouring
Body contouring addresses stubborn fat, skin laxity, or cellulite. Our board-certified physicians have been involved in clinical trials to develop new technology that provides superior treatment options. We offer treatments for cellulite, skin tightening, and fat reduction.
Fillers
Laser & Skin Surgery Center of New York’s filler treatments are always performed by board-certified physicians who have extensive experience with injectables. When you come to us for cosmetic care, you can be confident that you can safely achieve a perfectly natural look.
Haven't found
WHAT you are looking for?
patient approved
Trusted Worldwide
patient approved
Trusted Worldwide
By LASER & SKIN SURGERY CENTER OF NEW YORK® | © 2026 All Rights Reserved. | Privacy Policy |The information available on this web site is provided for informational purposes only. This information is not intended to replace a medical consultation where a physician's judgment may advise you about specific disorders, conditions and or treatment options. We hope the information will be useful for you to become more educated about your health care decisions. If you are vision-impaired or have some other impairment covered by the Americans with Disabilities Act or a similar law, and you wish to discuss potential accommodations related to using this website, please contact us at 212.941.5055.
*MDs perform 100% of all medical and cosmetic treatments.